CLINDABACTIN® 55 mg
check_circle In Stock
Usually ready in 4 hours —
Identification
- Name of the veterinary medicinal product
- CLINDABACTIN® 55 mg Chewable Tablets for Dogs and Cats
- Pharmaceutical form
- Compressed
Composition
- Active ingredients
-
- Clindamycin (as hydrochloride)
- Excipients
-
- Additional information
Clinical use
Indications for use by species
| Species) | Use |
|---|---|
|
Dogs : Treatment of infected wounds, abscesses and infections of the oral cavity, including periodontal disease, due to or associated with Staphylococcus spp., Streptococcus spp. (except Streptococcus faecalis ), Bacteroides spp., Fusobacterium necrophorum and Clostridium perfringens susceptible to clindamycin. Treatment of superficial pyoderma associated with clindamycin-sensitive Staphylococcus pseudintermedius . Treatment of osteomyelitis due to clindamycin-sensitive Staphylococcus aureus .
Cats : Treatment of infected wounds, abscesses and infections of the oral cavity, including periodontal disease, due to bacteria sensitive to clindamycin.
|
- Contraindications
-
Precautions and warnings
- Special warnings for each target species
-
None.
- Special precautions for safe use in target species
-
The chewable tablets are flavored. To prevent accidental ingestion, keep the tablets out of reach of animals.
The use of the veterinary medicinal product should be based on the performance of antibiograms of target bacteria isolated from the animal. If this is not possible, treatment should be based on local epidemiological information and the known susceptibility of the target pathogens at the local/regional level.
The veterinary medicinal product must be used in accordance with official national and regional policies regarding antibiotic therapy.
The use of the veterinary medicinal product outside the recommendations of the SPC may increase the prevalence of clindamycin-resistant bacteria and limit the effectiveness of treatment with lincomycin or macrolides due to potential cross-resistance.
Cross-resistance has been demonstrated with lincosamides (including clindamycin), erythromycin and other macrolides.
In some cases (localized or minor lesions; to prevent recurrence), superficial pyoderma can be treated topically. The need for and duration of systemic antimicrobial treatment should be determined by careful consideration of each individual case.
In case of prolonged treatment lasting more than one month, liver and kidney function and blood counts should be monitored regularly.
In animals with severe renal and/or very severe hepatic disorders, associated with severe metabolic abnormalities, the dose should be determined with caution, and their condition should be monitored by performing serum analyses during treatment with high doses of clindamycin.
The use of the veterinary drug is not recommended in newborns.
- Special precautions to be taken by the person administering the medication
-
Lincosamides (lincomycin, clindamycin, pirlimycin) can cause hypersensitivity reactions (allergies). People with a known hypersensitivity to lincosamides should avoid all contact with the veterinary medicinal product.
Wash your hands after handling the tablets.
Accidental ingestion may cause gastrointestinal effects, such as abdominal pain and diarrhea. Precautions should be taken to avoid accidental ingestion.
To limit the risk of accidental ingestion by children, do not remove the tablets from the blister pack before administering them to the animal. Return any partially used tablets to the blister pack and the box and use them for the next administration.
In case of accidental ingestion, especially by a child, seek medical advice immediately and show the doctor the package leaflet or label.
- Special precautions regarding environmental protection
-
None.
- Other precautions
-
None.
Interactions and special cases
- Use during pregnancy, lactation, or egg-laying
-
Gestation and lactation :
Although studies with high doses in rats suggest that clindamycin is not teratogenic and does not significantly affect the reproductive performance of males and females, safety in pregnant female dogs/cats or dogs/cats intended for breeding has not been established.
Clindamycin crosses the placental barrier and the blood-mammary barrier.
Treatment of lactating females can cause diarrhea in puppies and kittens.
Use should only occur after a benefit/risk assessment has been established by the responsible veterinarian.
- Drug interactions and other forms of interaction
-
Clindamycin hydrochloride has neuromuscular blocking properties that may enhance the action of other curarizing agents. This veterinary medicinal product should be used with caution in animals receiving such agents.
Aluminum salts and hydroxides, kaolin, and aluminum-magnesium-silicate complex can reduce the absorption of lincosamides. These gastrointestinal protectants should be administered at least 2 hours before clindamycin.
Clindamycin should not be used concurrently with, or immediately after, erythromycin or other macrolides to avoid macrolide-induced clindamycin resistance.
Clindamycin may reduce plasma levels of cyclosporine and expose to a risk of lack of activity.
When clindamycin and aminoglycosides (e.g. gentamicin) are used simultaneously, the risk of adverse interactions (acute renal failure) cannot be excluded.
Clindamycin should not be used concomitantly with chloramphenicol or macrolides, as they have a reciprocal antagonistic effect on their site of action at the 50S ribosome subunit.
- Antimicrobials and antiparasitics: combating resistance
-
None.
Side effects and overdose
- Overdose (symptoms, emergency procedures, antidote)
- Major incompatibility
- Side effects
-
Dogs and cats:
Uncommon
(1 to 10 animals / 1,000 animals treated):
Vomiting, diarrhea, imbalance of gastrointestinal flora Clindamycin causes overgrowth of non-susceptible organisms, such as resistant yeasts and clostridium. In case of superinfection, appropriate measures should be taken according to the clinical situation.
It is important to report adverse reactions. Reporting allows for ongoing monitoring of the safety of a veterinary medicinal product. Reports should be sent, preferably via a veterinarian, either to the marketing authorization holder or their local representative, or to the competent national authority via the national reporting system. See the package leaflet for the respective contact details.
Dosage
| Species) | Dosage |
|---|---|
|
|
1. For the treatment of infected wounds, abscesses, and infections of the oral cavity, including periodontal disease, in dogs and cats, administer either: - 5.5 mg of clindamycin/kg of body weight every 12 hours for 7-10 days, i.e. - 11 mg of clindamycin/kg of body weight every 24 hours for 7-10 days. If no clinical response is obtained within 4 days, review the diagnosis.
2. For the treatment of superficial pyoderma in dogs, administer either: - 5.5 mg of clindamycin/kg of body weight every 12 hours, i.e. - 11 mg of clindamycin/kg of body weight every 24 hours. A 21-day course of treatment is generally recommended for canine superficial pyoderma, with the duration of treatment being shortened or prolonged based on clinical evaluation.
3. For the treatment of osteomyelitis in dogs, administer: - 11 mg of clindamycin/kg of body weight every 12 hours for at least 28 days. If no clinical response is obtained within 14 days, stop treatment and review the diagnosis.
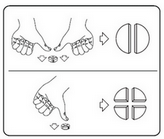
To ensure proper dosage, body weight must be determined as accurately as possible to avoid underdosing. The tablets can be divided into 2 or 4 equal parts to allow for correct dosage. Place the tablet on a flat surface, scored side up and convex (rounded) side down.
2 equal parts: press on both sides of the tablet with your thumbs. 4 equal parts: press in the center of the tablet with your thumb.
|
Waiting time
Preservation and storage
- Storage temperature
- Not specified
- Special storage precautions as appropriate
-
Lincosamides (lincomycin, clindamycin, pirlimycin) may cause hypersensitivity reactions (allergies). People with a known hypersensitivity to lincosamides should avoid all contact with the veterinary medicinal product.
Wash your hands after handling the tablets.
Accidental ingestion may cause gastrointestinal effects, such as abdominal pain and diarrhea. Precautions should be taken to avoid accidental ingestion.
To limit the risk of accidental ingestion by children, do not remove the tablets from the blister pack before administering them to the animal. Return any partially used tablets to the blister pack and the carton and use them for the next administration.
In case of accidental ingestion, especially by a child, seek medical advice immediately and show the doctor the package leaflet or the label. - Special precautions to be taken during disposal
-
Do not dispose of medications down the drain or in household waste.
Use the take-back arrangements put in place for the disposal of any unused veterinary medicinal product or waste derived therefrom, in accordance with local requirements and any national collection scheme applicable to the veterinary medicinal product concerned.
Commercial information
| Presentation | Accessible to the group | Drug classification in terms of dispensing | Marketing authorization number |
|---|---|---|---|
| CLINDABACTIN® 55 mg Chewable Tablets for Dogs and Cats Box of 10 Tablets | Yes | Subject to prescription | FR/V/9475988 6/2019 |
Revision information
- Date of revision of the notice
Responsibilities
- Responsible party (Marketing authorization holder)
- DECHRA Veterinary Products SAS
- Responsible (Marketing Officer)
- DECHRA Veterinary Products SAS
- Responsible (Pharmacovigilance Officer)
- DECHRA Veterinary Products SAS

102 Abdullah Bin Masoud Street
13036 Al Kuwayt KW-HA
Kuwait
check_circle Pickup available, usually ready in 4 hours